Voluntis and WellDoc®, two of the industry’s leading digital therapeutics companies, announced a commercial agreement aimed at combining Voluntis’ insulin titration technology with WellDoc’s extensive digital coaching platform to create a holistic digital diabetes management solution for patients, providers, health systems and health plans.
Voluntis developed Insulia®, an FDA-cleared, prescription-only digital companion for people and their care teams using basal insulin to treat type 2 diabetes. It provides automated basal insulin dose recommendations for people with type 2 diabetes while enabling the health care team to remotely monitor progress.
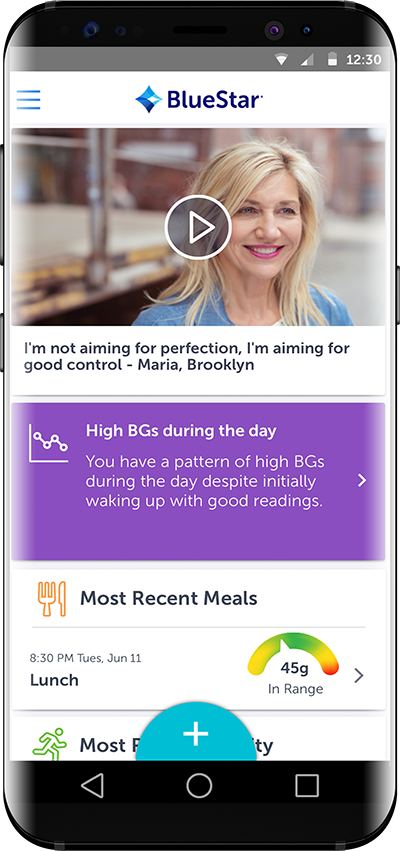
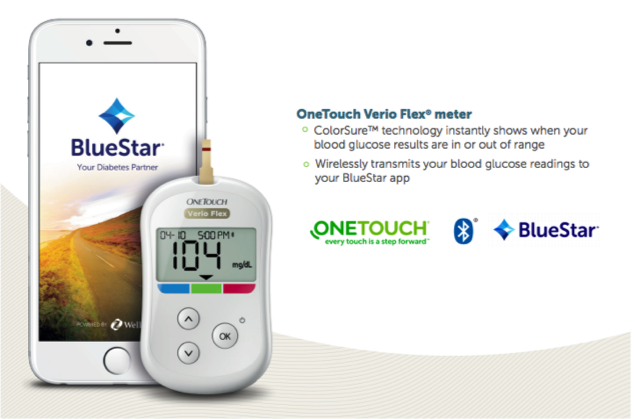
The developer of BlueStar® – an FDA-cleared, digital therapeutic for individuals with type 2 diabetes – WellDoc is the first digital health company in the US to conduct randomized clinical trials that demonstrate significant clinical outcomes, as published in multiple peer-reviewed journals and presented at leading industry conferences. The clinical evidence shows a 1.7 to 2.0- point mean A1C reduction for individuals living with type 2 diabetes who used BlueStar. 1 BlueStar provides real-time and timely individualized coaching and support, as well as educational tools that are actionable and personal for individuals living with type 2 diabetes.
The pioneering companies maintain a shared vision of how digital technologies can positively impact the health of patients. In order to realize this goal, Voluntis and WellDoc are committed to clinical validation of their solutions, rigorous adherence to regulatory requirements, working with stakeholders to drive utilization and reimbursement, and collaboration with industry partners that complement each company’s respective solutions.
Read More